We reverse and relieve the immunosuppressive nature of the tumor microenvironment
Cancer immunotherapy is a highly promising therapeutic intervention and is currently aimed at almost every major cancer. Cancer treatment by immune checkpoint inhibitor (CPI) extends survival; however, response rates from CPI continue to be low primarily due to the immunosuppressive tumor microenvironment (TME).
We are leveraging the power of the Elite Responder’s immune system to discover & develop fully-human, immunomodulatory antibodies from patients who have achieved exceptional responses to CPI therapies. We focus on antibodies that reverse and relieve immunosuppression in the tumor microenvironment to enhance responses of CPI therapy to greater cures.
We utilize a clinically validated Technology Platform to discover human antibodies to multiple high value targets associated with immunosuppressive myeloid biology and develop therapeutics to relieve immunosuppression of the TME and augment adaptive immune activity to make “cold” tumors “hot”.
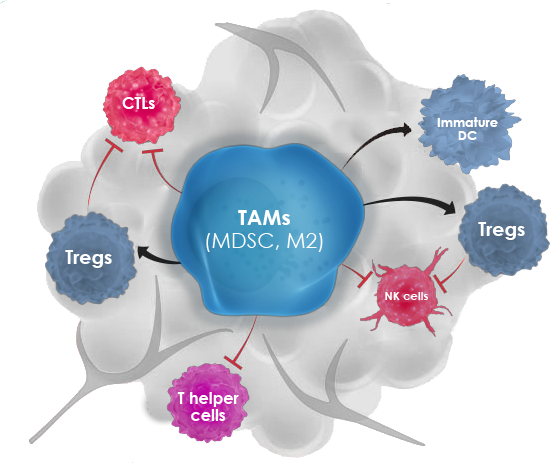
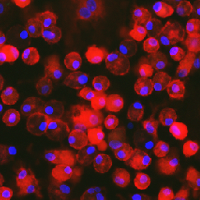
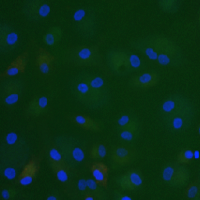
Tumor-associated macrophages (TAMs) represent a major constituent of the leukocytic infiltrate in the TME. During tumor progression, TAMs are polarized from a M1-like anti-tumor to a M2-like pro-tumor phenotype. M2 TAMs contribute to CPI therapy resistance by actively inhibiting anti-tumor T-cell proliferation and cytotoxic activity, by promoting expansion of pro-tumorigenic T regulatory cells, thereby dampening the host immune responses against the tumor. In addition, these M2 TAMs display tumor-promoting functions by facilitating tumor proliferation and survival, angiogenesis, and metastasis. High infiltration of TAMs generally predicts unfavorable prognosis, and reduction or repolarization of suppressive myeloid cells enhances clinical responses to CPI therapy.
OncoResponse lead antibody, OR2805 was isolated from a cancer patient who achieved complete response with CPI treatment. OR2805 antibody target is highly specific to M2-like TAMs and not expressed on other hematopoietic cells. Our antibodies reprogram M2-like TAMs to relieve immunosuppression of the TME and leads to increased T-cell activation and proliferation. This reprogramming of TAMs may therefore enhance clinical responses to immunotherapy.
Target Binding