OncoResponse's antibodies to key targets reverse and relieve immunosuppression in the tumor microenvironment in the effort to enhance responses from immunotherapy for greater cures. See our platform for our ongoing discovery of rare human antibodies derived from Elite Responders.
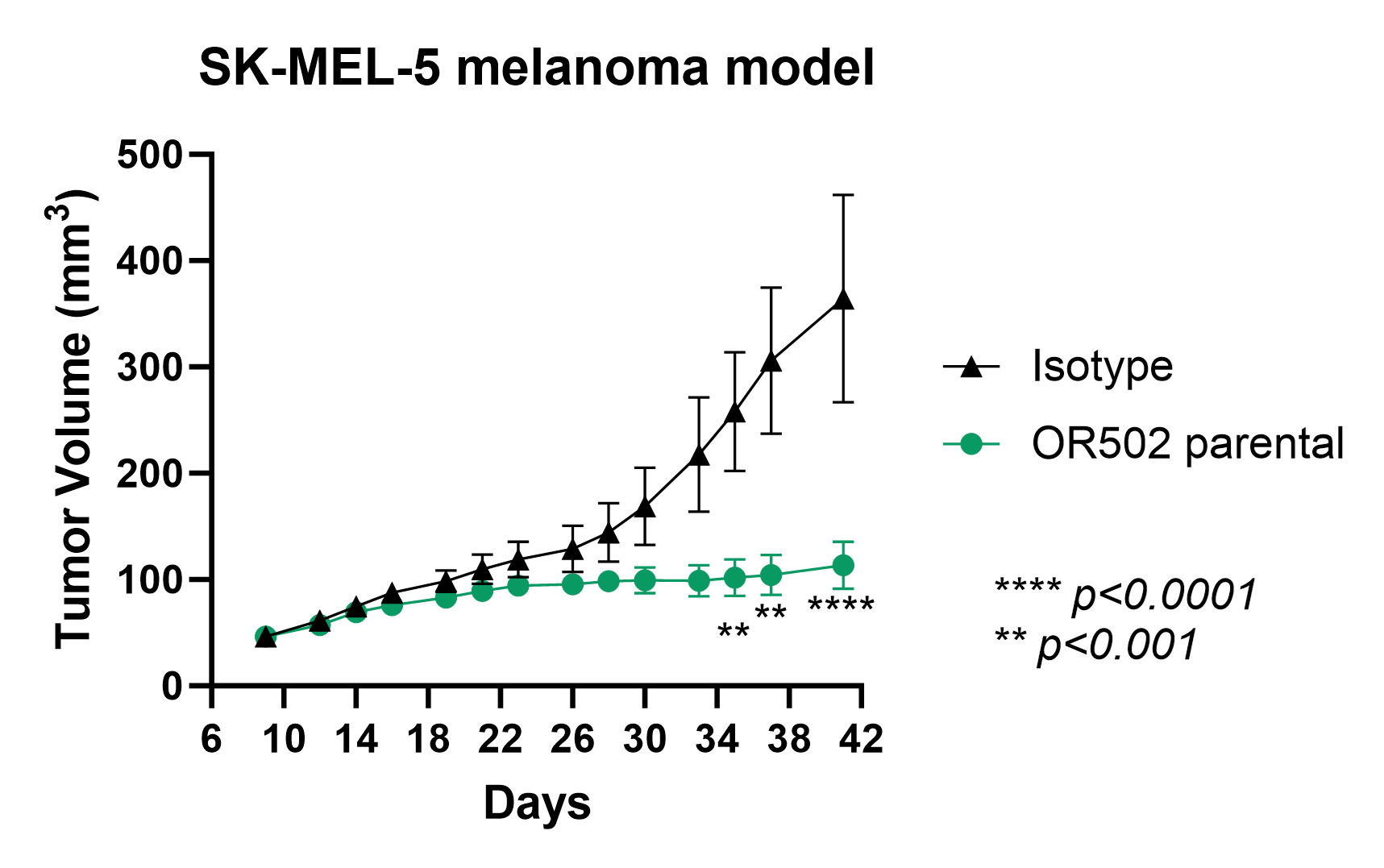
OR502 demonstrates superior efficacy in SK-MEL-5 melanoma xenograft model in humanized NSG-SGM3 mice
OR502 has a potential to treat several different tumor types.
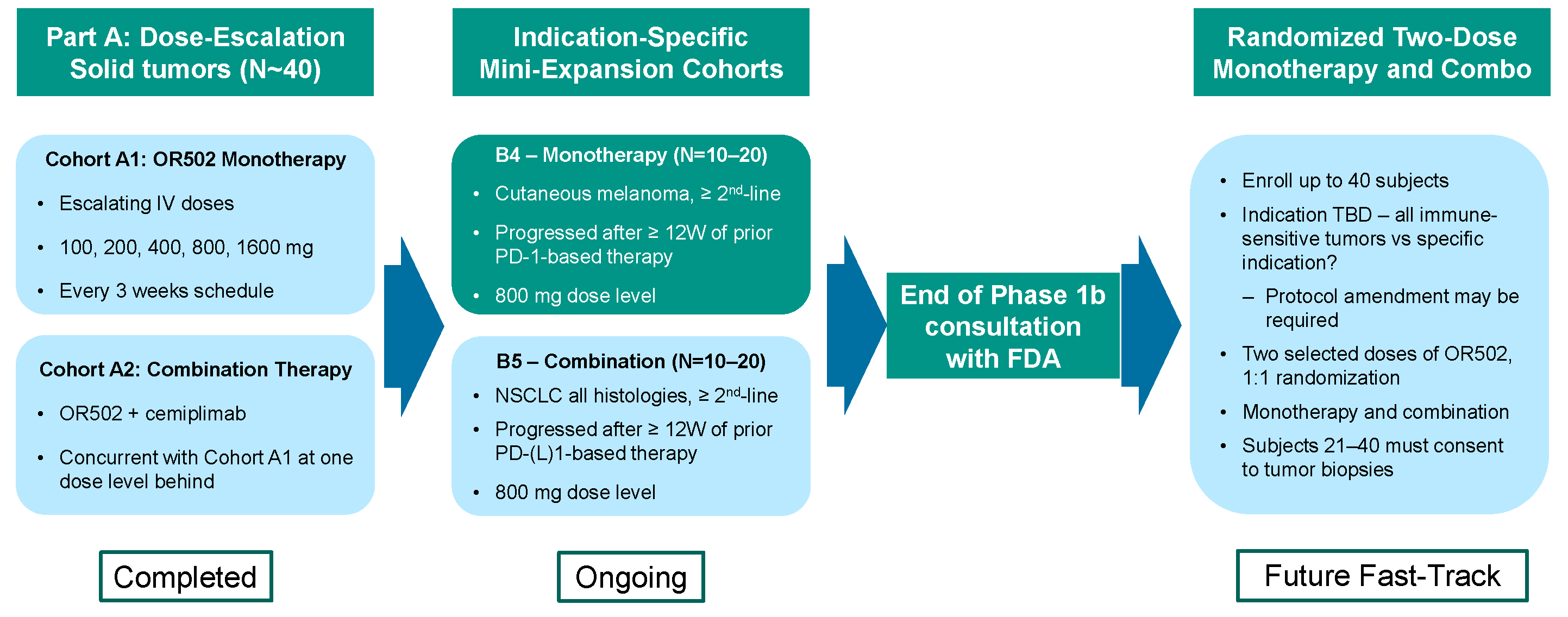
We completed a Part A dose-escalation study in subjects with advanced solid tumors. This study demonstrated strong results for safety, receptor occupancy, pharmacokinetics, disease control and potential drug efficacy. These findings enabled us to set the Phase 2 dose level and initiate the next set of clinical studies.
OR502 has begun indication-specific clinical studies in melanoma (monotherapy) and NSCLC (in combination with cemiplimab).
OR502-101 Study Design
LILRB2 causes immunosuppression via multiple mechanisms.
LILRB2 not only binds to HLA-G on cancer cells, but also to MHC Class I molecules in cis- and trans-orientation. OR502 binds to a distinct epitope and blocks engagement of LILRB2 to both HLA-G and MHC Class I molecules.
OR502 is differentiated from other LILRB2 antibodies:
•While other LILRB2 antibodies block LILRB2 interactions with HLA-G, only OR502 can also block interactions with MHC Class I molecules to free up MHC for interactions with TCRs required for activation and proliferation of T cells.
•FcgRs contribute to myeloid cell activation. While other LILRB2 antibodies are IgG4 isotype, OR502 is an IgG1 and can co-engage immunostimulatory FcgRs on myeloid cells for superior reprograming to inflammatory phenotype. This is an important differentiator from other LILRB2 antibodies.
In summary, OR502 targets multiple inhibitory mechanisms of LILRB2 and engages an immunostimulatory signal making it a best-in-class antibody. This is supported by superior clinical data with a high Disease Control Rate of 65% in monotherapy, compelling OncoResponse to continue clinical trials in melanoma and NSCLC under our Phase 1b efficacy confirmatory studies.
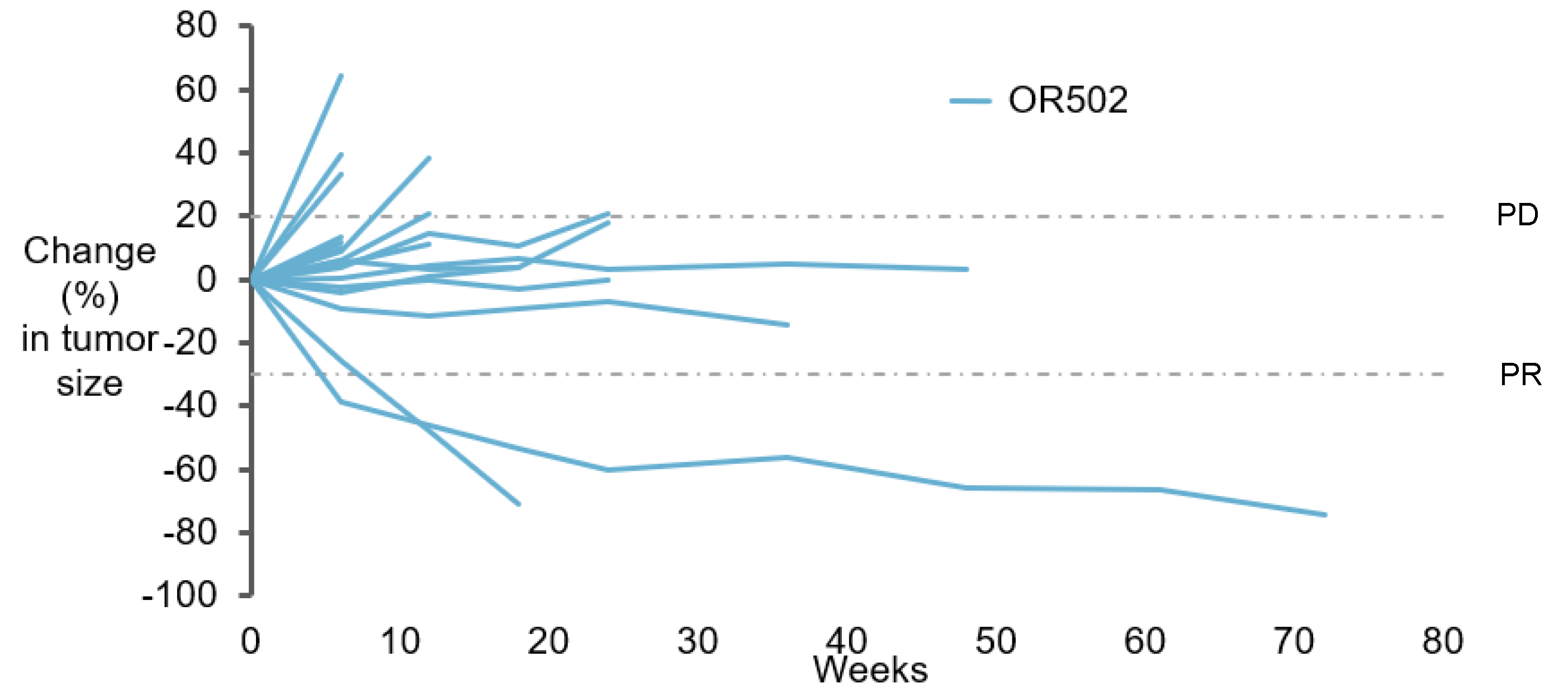
A1 Monotherapy - Most Patients had Disease Control at First Scan